The only non-mRNA, recombinant protein-based COVID-19 vaccine approved by the FDA
NUVAXOVID works differently
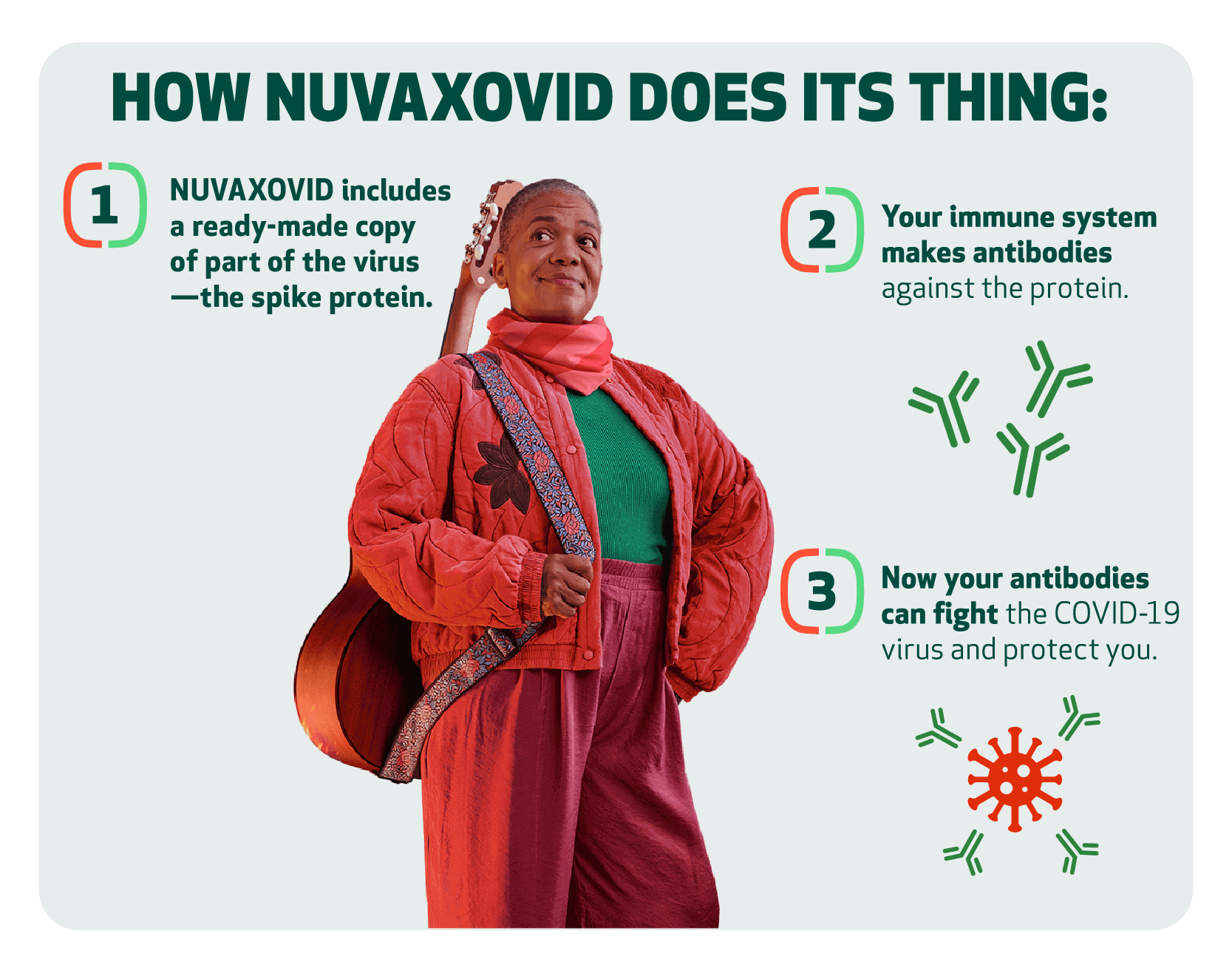
NUVAXOVID is a recombinant protein-based vaccine that works differently than mRNA vaccines.
mRNA vaccines require our cells to make a protein like the one in the virus—then your immune system can learn to recognize it as a target. NUVAXOVID delivers the protein ready-made, directly to your immune system.
It's an established and precise way to help your body recognize and fight disease. Recombinant technology creates copies of just part of a virus to trigger your immune system to respond.
This is the same tried-and-true technology widely used in certain seasonal flu vaccines
From the proven name in protection
NUVAXOVID is brought to you by Sanofi, which has over a century of expertise in developing life-saving vaccines. It's why over 500 million people around the world each year trust Sanofi vaccines to help protect them.
Talk to your pharmacist or doctor about NUVAXOVID, the only non-mRNA, recombinant protein-based COVID-19 vaccine
Important Safety Information
You or your child should not get NUVAXOVID if you had a severe allergic reaction to a previous dose of NUVAXOVID or to any of its ingredients.
There is a remote chance that the vaccine could cause a severe allergic reaction, which would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include: difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body and/or dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received NUVAXOVID. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine: chest pain; shortness of breath; feelings of having a fast-beating, fluttering, or pounding heart.
Other side effects reported in clinical trials and/or post-approval use of NUVAXOVID include: Injection site reactions (pain/tenderness, swelling, redness and itching), fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills, allergic reactions such as hives and swelling of the face, swollen lymph nodes, paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), and hypoesthesia (decreased feeling or sensitivity, especially in the skin)
Indication
NUVAXOVID is a vaccine to protect against COVID-19 for people who are:
- 65 years of age and older, or
- 12 years through 64 years of age at high risk for severe COVID-19
Vaccination with NUVAXOVID may not protect all people who receive the vaccine.
NUVAXOVID does not contain SARS-CoV-2, the virus that causes COVID-19. NUVAXOVID cannot give you or your child COVID-19.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
Important Safety Information
You or your child should not get NUVAXOVID if you had a severe allergic reaction to a previous dose of NUVAXOVID or to any of its ingredients.
There is a remote chance that the vaccine could cause a severe allergic reaction, which would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include: difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body and/or dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received NUVAXOVID. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine: chest pain; shortness of breath; feelings of having a fast-beating, fluttering, or pounding heart.
Other side effects reported in clinical trials and/or post-approval use of NUVAXOVID include: Injection site reactions (pain/tenderness, swelling, redness and itching), fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills, allergic reactions such as hives and swelling of the face, swollen lymph nodes, paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), and hypoesthesia (decreased feeling or sensitivity, especially in the skin)
Indication
NUVAXOVID is a vaccine to protect against COVID-19 for people who are:
- 65 years of age and older, or
- 12 years through 64 years of age at high risk for severe COVID-19
Vaccination with NUVAXOVID may not protect all people who receive the vaccine.
NUVAXOVID does not contain SARS-CoV-2, the virus that causes COVID-19. NUVAXOVID cannot give you or your child COVID-19.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
Important Safety Information
You or your child should not get NUVAXOVID if you had a severe allergic reaction to a previous dose of NUVAXOVID or to any of its ingredients.
There is a remote chance that the vaccine could cause a severe allergic reaction, which would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include: difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body and/or dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received NUVAXOVID. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine: chest pain; shortness of breath; feelings of having a fast-beating, fluttering, or pounding heart.
Other side effects reported in clinical trials and/or post-approval use of NUVAXOVID include: Injection site reactions (pain/tenderness, swelling, redness and itching), fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills, allergic reactions such as hives and swelling of the face, swollen lymph nodes, paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), and hypoesthesia (decreased feeling or sensitivity, especially in the skin)
Indication
NUVAXOVID is a vaccine to protect against COVID-19 for people who are:
- 65 years of age and older, or
- 12 years through 64 years of age at high risk for severe COVID-19
Vaccination with NUVAXOVID may not protect all people who receive the vaccine.
NUVAXOVID does not contain SARS-CoV-2, the virus that causes COVID-19. NUVAXOVID cannot give you or your child COVID-19.
Learn more about Sanofi's commitment to fighting counterfeit drugs.