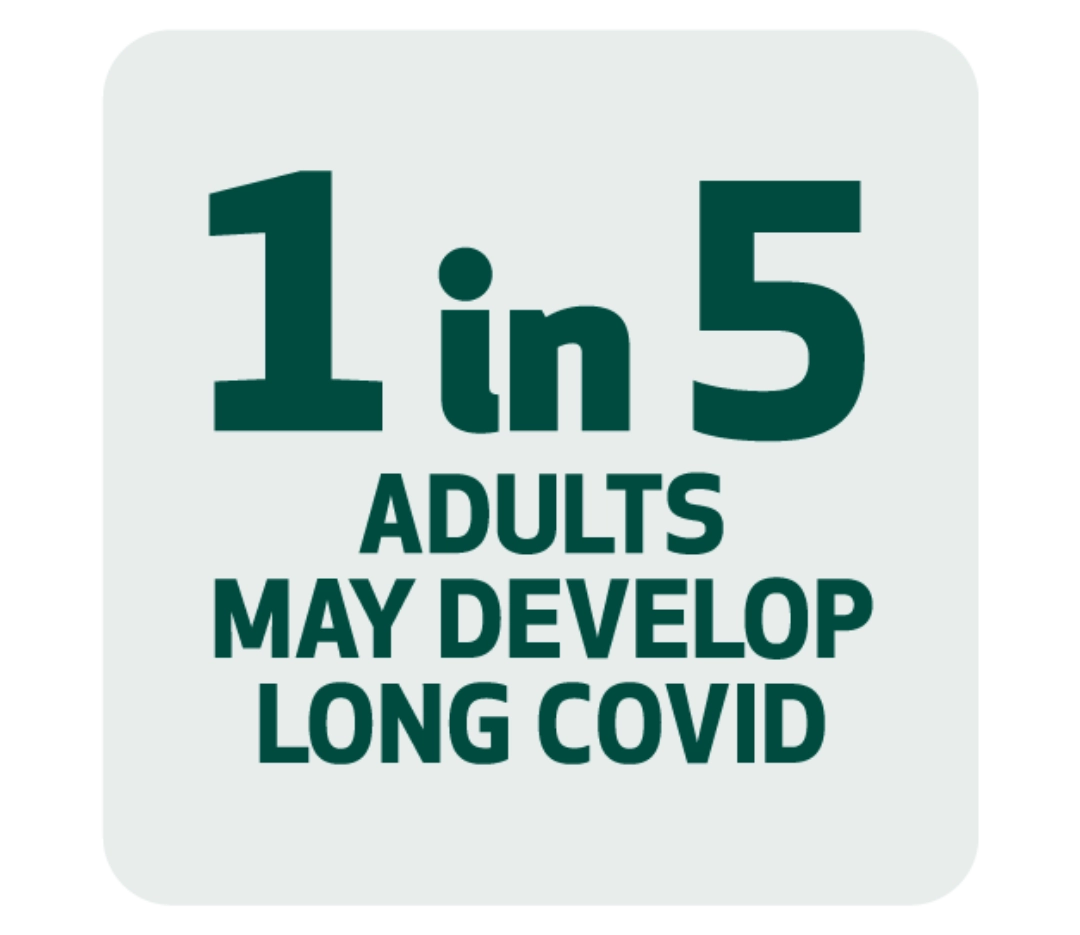COVID-19 isn't over. Your protection shouldn't be either
COVID-19 is still a serious and ongoing threat
- The CDC estimates that between October 2024 and June 2025 COVID-19 has caused 270,000-440,000 hospitalizations and 32,000-51,000 deaths
- About 1 in 5 adults hospitalized due to COVID-19 were admitted to the intensive care unit October 2023 - May 2024
Who's at risk?
~75% of American adults have at least one risk factor for getting seriously ill from COVID-19 including:
- People who are unvaccinated
- Adults 65+
- People with comorbidities like chronic lung disease, cardiovascular disease, chronic liver disease, severe obesity, and diabetes
Why staying up to date on your vaccination matters
The virus that causes COVID-19 keeps evolving. To stay protected against the ongoing threat, the CDC recommends a COVID-19 vaccine every year for people who are at higher risk for complications. NUVAXOVID is a COVID-19 vaccine that is designed for seasonal vaccination.
Long COVID can be a lasting burden
- About 1 in 5 adults who have had COVID-19 may develop long COVID, according to a 2022 study. This is a serious, chronic illness with symptoms that include fatigue, brain fog, and serious respiratory or heart issues. These symptoms can last weeks, months or even years after the initial illness.

Talk to your pharmacist or doctor about NUVAXOVID, the only non-mRNA, recombinant protein-based COVID-19 vaccine
FAQs About COVID-19
A: COVID-19 is still circulating and causing serious illness, especially in older adults and people with health conditions. The CDC estimates that between October 2024 and June 2025 COVID-19 has caused 270,000-440,000 hospitalizations and 32,000-51,000 deaths. It can also cause long COVID, with symptoms that can last—or even appear—years later. Staying protected matters, even if we're no longer in a pandemic.
A: A pandemic is when a new virus spreads quickly across the world, like COVID-19 did in 2020. An epidemic is a sudden outbreak that spreads rapidly in a specific area. Endemic means the virus is here to stay. It’s still a serious, and sometimes deadly threat, but we’ve learned how to manage it—like we do with the seasonal flu.
A: The virus continues to evolve, and your immunity can fade over time. That’s why the CDC recommends yearly vaccination for some people—to stay protected against the latest variants and reduce your risk of severe illness or long COVID.
A: All vaccines in the U.S meet the FDA’s high standards for efficacy and safety. This includes going through 3 phases of clinical trials. See more details at the CDC website.
A: It’s still possible to get infected. But unvaccinated people are more likely to get COVID-19 and much more likely to be hospitalized and to die from COVID-19, compared to people who are up to date with their COVID-19 vaccinations. A vaccination also reduces the severity of the illness if you do get COVID-19.
A: Vaccination remains the best available protection against the most severe outcomes of COVID-19, including hospitalization and death, even if you have previously been infected. Vaccination can also help reduce the severity of COVID-19 if you do get infected.
A: Long COVID is a serious, chronic illness that occurs after a COVID-19 infection. More than 200 possible symptoms of long COVID have been identified, including fatigue, brain fog, and serious respiratory or heart issues. These symptoms can last weeks, months, or even years after the initial illness.
Pregnancy
A: If you are pregnant or were recently pregnant, you are more likely to get very sick from COVID-19, compared to those who are not pregnant. Additionally, if you get COVID-19 while you are pregnant, you, your pregnancy, and your baby are at increased risk of complications arising from serious illness.
A: If you are pregnant or breastfeeding, discuss your options with your healthcare provider. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to NUVAXOVID during pregnancy. Women who are vaccinated with NUVAXOVID during pregnancy are encouraged to enroll in the registry by visiting https://c-viper.pregistry.com
Important Safety Information
You or your child should not get NUVAXOVID if you had a severe allergic reaction to a previous dose of NUVAXOVID or to any of its ingredients.
There is a remote chance that the vaccine could cause a severe allergic reaction, which would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include: difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body and/or dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received NUVAXOVID. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine: chest pain; shortness of breath; feelings of having a fast-beating, fluttering, or pounding heart.
Other side effects reported in clinical trials and/or post-approval use of NUVAXOVID include: Injection site reactions (pain/tenderness, swelling, redness and itching), fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills, allergic reactions such as hives and swelling of the face, swollen lymph nodes, paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), and hypoesthesia (decreased feeling or sensitivity, especially in the skin)
Indication
NUVAXOVID is a vaccine to protect against COVID-19 for people who are:
- 65 years of age and older, or
- 12 years through 64 years of age at high risk for severe COVID-19
Vaccination with NUVAXOVID may not protect all people who receive the vaccine.
NUVAXOVID does not contain SARS-CoV-2, the virus that causes COVID-19. NUVAXOVID cannot give you or your child COVID-19.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
Important Safety Information
You or your child should not get NUVAXOVID if you had a severe allergic reaction to a previous dose of NUVAXOVID or to any of its ingredients.
There is a remote chance that the vaccine could cause a severe allergic reaction, which would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include: difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body and/or dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received NUVAXOVID. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine: chest pain; shortness of breath; feelings of having a fast-beating, fluttering, or pounding heart.
Other side effects reported in clinical trials and/or post-approval use of NUVAXOVID include: Injection site reactions (pain/tenderness, swelling, redness and itching), fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills, allergic reactions such as hives and swelling of the face, swollen lymph nodes, paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), and hypoesthesia (decreased feeling or sensitivity, especially in the skin)
Indication
NUVAXOVID is a vaccine to protect against COVID-19 for people who are:
- 65 years of age and older, or
- 12 years through 64 years of age at high risk for severe COVID-19
Vaccination with NUVAXOVID may not protect all people who receive the vaccine.
NUVAXOVID does not contain SARS-CoV-2, the virus that causes COVID-19. NUVAXOVID cannot give you or your child COVID-19.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
Important Safety Information
You or your child should not get NUVAXOVID if you had a severe allergic reaction to a previous dose of NUVAXOVID or to any of its ingredients.
There is a remote chance that the vaccine could cause a severe allergic reaction, which would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include: difficulty breathing, swelling of the face and throat, a fast heartbeat, a bad rash all over the body and/or dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received NUVAXOVID. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine: chest pain; shortness of breath; feelings of having a fast-beating, fluttering, or pounding heart.
Other side effects reported in clinical trials and/or post-approval use of NUVAXOVID include: Injection site reactions (pain/tenderness, swelling, redness and itching), fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills, allergic reactions such as hives and swelling of the face, swollen lymph nodes, paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), and hypoesthesia (decreased feeling or sensitivity, especially in the skin)
Indication
NUVAXOVID is a vaccine to protect against COVID-19 for people who are:
- 65 years of age and older, or
- 12 years through 64 years of age at high risk for severe COVID-19
Vaccination with NUVAXOVID may not protect all people who receive the vaccine.
NUVAXOVID does not contain SARS-CoV-2, the virus that causes COVID-19. NUVAXOVID cannot give you or your child COVID-19.
Learn more about Sanofi's commitment to fighting counterfeit drugs.